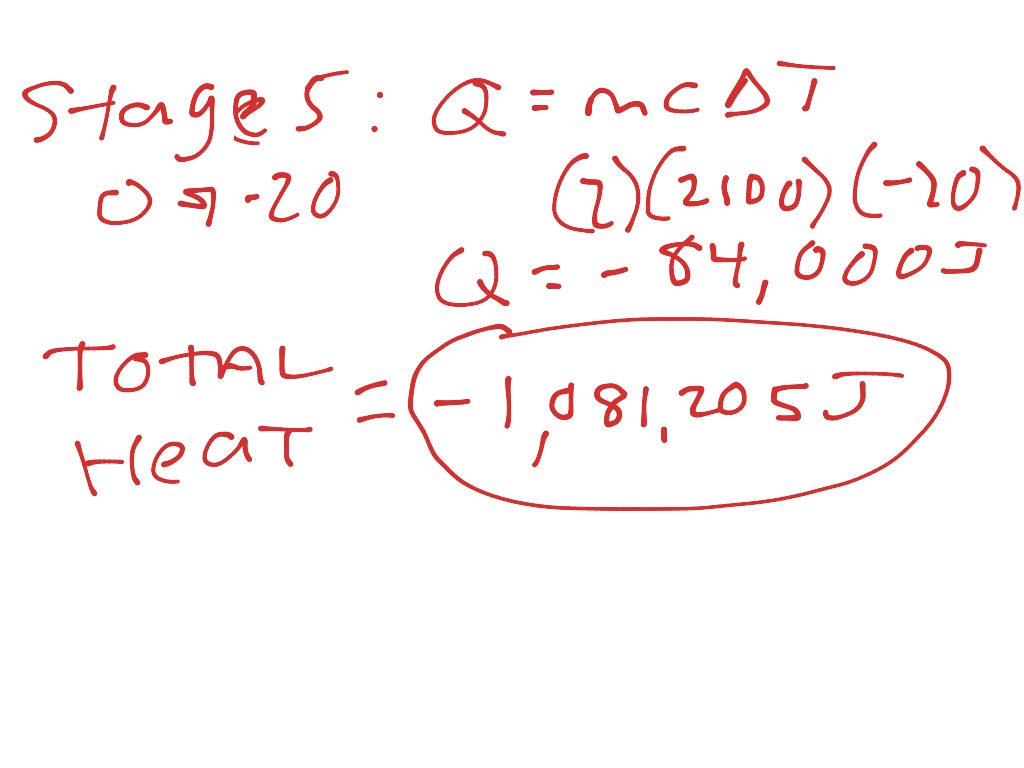Change Of Phase Examples . The temperature of the ice rises. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert.
from www.showme.com
The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The temperature of the ice rises.
Change of Phase example 1 Science, Chemicalreactions, Heat ShowMe
Change Of Phase Examples The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. The temperature of the ice rises. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of.
From www.britannica.com
Phase Definition & Facts Britannica Change Of Phase Examples The temperature of the ice rises. Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Learn what is phase change or phase transition and the way in which they. Change Of Phase Examples.
From quizlet.com
Phase Change Diagram Diagram Quizlet Change Of Phase Examples The temperature of the ice rises. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Phase transitions describe matter changing from one state to another, such as from the solid to. Change Of Phase Examples.
From studylib.net
phase changes Change Of Phase Examples These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. The temperature of the ice rises. Learn what is phase change or. Change Of Phase Examples.
From www.thoughtco.com
List of Phase Changes Between States of Matter Change Of Phase Examples The temperature of the ice rises. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. Phase transitions describe matter changing from. Change Of Phase Examples.
From www.slideshare.net
Phase changes Change Of Phase Examples Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. The temperature of the ice rises. Learn what is phase change or phase transition and the way in which they. Change Of Phase Examples.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry Change Of Phase Examples Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you. Change Of Phase Examples.
From www.proferecursos.com
Phase changes of matter Change Of Phase Examples Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. The temperature of the ice rises. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Learn what is phase. Change Of Phase Examples.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free Change Of Phase Examples Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The temperature of the ice rises. Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. These processes require energy, above and beyond that needed to change the temperature to. Change Of Phase Examples.
From www.seekpng.com
Types Of Phase Change Phase Transition (2364x1300), Png Download Change Of Phase Examples Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The temperature of the ice rises. Phase transitions describe matter changing from one state to another, such as from the solid. Change Of Phase Examples.
From mungfali.com
Stages Of Change Diagram Change Of Phase Examples The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Learn what is phase change or phase transition and the way in. Change Of Phase Examples.
From www.youtube.com
Phase Change Diagrams YouTube Change Of Phase Examples The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and. Change Of Phase Examples.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Change Of Phase Examples These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The temperature of the ice rises. Phase transitions describe matter changing from one state to another, such as from the solid to. Change Of Phase Examples.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Change Of Phase Examples These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. The temperature of the ice rises. The following paragraphs will define. Change Of Phase Examples.
From www.youtube.com
Phases of Matter and the Phase Changes YouTube Change Of Phase Examples The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. The temperature of the ice rises. Learn what is phase change or. Change Of Phase Examples.
From opentextbc.ca
Phase Changes Basic HVAC Change Of Phase Examples Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing and melting of. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing,. Change Of Phase Examples.
From www.slideshare.net
Phase changes Change Of Phase Examples Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Learn what is phase change or phase transition and the way in which they occur, the phenomenon of boiling, condensation, evaporation, freezing. Change Of Phase Examples.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Change Of Phase Examples Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. The following paragraphs will define and describe the six phase changes, or changes in state, through which these physical forms of matter can interconvert. Learn what is phase change or phase transition and the. Change Of Phase Examples.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 Change Of Phase Examples Phase transitions describe matter changing from one state to another, such as from the solid to the liquid phase, or the liquid to the gas phase. These processes require energy, above and beyond that needed to change the temperature to one where the processes occur, which you can easily calculate. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation,. Change Of Phase Examples.